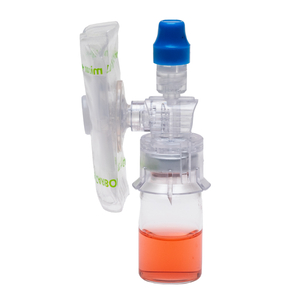
ProSeal CSTD
COMPLIANCE
INTERNATIONAL REGULATORY STANDARDS
European regulatory bodies, including the European Medicines Agency (EMA) and national authorities, are actively engaged in developing and implementing comprehensive regulations and standards for CSTDs. These standards are designed to ensure the safety, efficacy, and quality of CSTDs across the European market. The harmonization of regulatory requirements promotes consistent and standardized use of CSTDs in diverse healthcare settings.
NIOSH REQUIREMENTS
PRoSeal™ CSTDs meet the NIOSH requirements for closed system transfer devices, mechanically preventing the transfer of environmental contaminants and the escape of hazardous drugs or vapor concentrations. For instance, ProSeal™– ToxiSeal Vial Adaptors, are equipped with an external balloon, in addition to a 0.1 micron sterile filter and activated carbon filters.
PHARMACEUTICAL SAFETY STANDARDS
ProSeal™ CSTDs are FDA ONB & FPA code cleared and compliant with USP<797> and USP<800> standards, demonstrating their commitment to pharmaceutical safety regulations.
TESTS
PROVEN MICROBIAL INGRESS PREVENTION
ProSeal™ CSTDs have been rigorously tested and confirmed to prevent microbial ingress for at least 168 hours after 10 activations, ensuring long lasting effectiveness.
ERGONOMICS
USER-FRIENDLY PUSH/PULL ACTION
ProSeal™ Devices feature a quick and simple Push/Pull action, eliminating the need for repetitive rotation and reducing the risk of RSI-type injuries (Repetitive Strain Injuries).
OPTIMIZATION
COST EFFECTIVE & EFFICIENT
Beyond safety, the design of ProSeal™ CSTDs enables efficient manufacturability, resulting in competitive pricing.
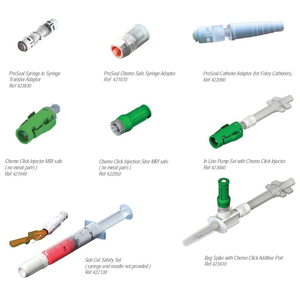
ProSeal™ Accessories
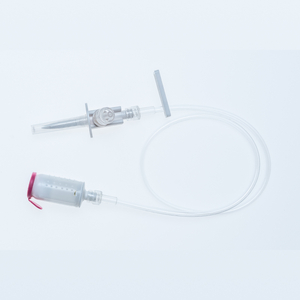
ProSeal™ In Line Pump Set

ProSeal™ Bag Spike

ProSeal™ Administration Sets

ProSeal™ Bag Adaptor

ProSeal™ Injection Site

ProSeal™ Injectors
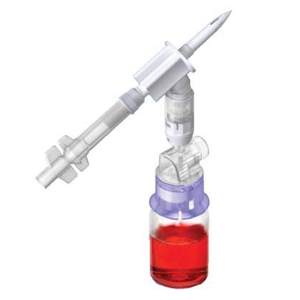
ProSeal™ Add Mixture Device (ADRx)